If you did not get the chance earlier this month to attend one of Anne Wilson’s presentations on Current Trends in FDA Inspections, you missed out on some interesting information relevant to 483 reports. In particular, did you know that design controls was the most commonly cited CFR section in 483 reports in 2014? This does not come as much surprise to QA Consulting as we often find that companies make mistakes when implementing design controls, including conducting insufficient reviews and starting design controls at a late stage in the product lifecycle. In this article, we begin our series on design controls with advice on avoiding common mistakes.
Design Controls Series: How to Excel by Avoiding Common Mistakes
A recent analysis of FDA 483s from fiscal year 2014 discovered that the most common 483 observation was related to design control (21 CFR 820.30) deficiencies. Medical device manufacturers often misunderstand the terminology, and FDA’s expectations regarding design controls. The design control requirements apply to FDA Class II and Class III medical devices, as well as a small number of Class I devices. Keep in mind that in the EU and Canada, all medical devices must comply with design control requirements of ISO 13485.
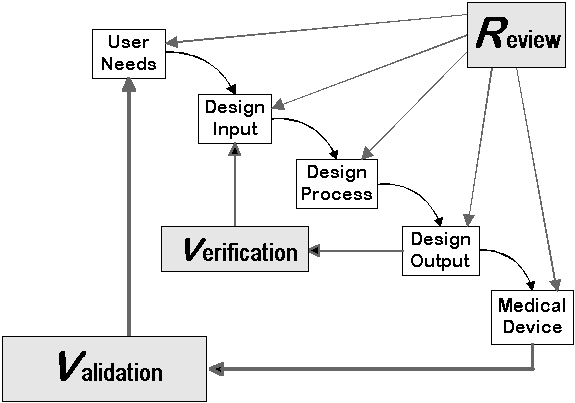
To avoid common mistakes manufacturers make, consider the five suggestions below during your next design project:
1. Review the design plan. A documented design plan should be developed and approved. The design process is iterative, and plans will likely change. Be sure to capture changes in the plan through periodic reviews and updates.
2. Define quantitative, verifiable design inputs. Design inputs should be measurable and verifiable. For example, portability of Device XYZ might be a user or marketing requirement. Appropriate design inputs for this type of user requirement could be:
a. Device XYZ shall weigh less than 2.0 lbs
b. Device XYZ shall be smaller than 5.0” x 4.0” x 2.0”
3. Initiate risk management early. Integrate a risk management process as early as possible in the design, preferably during the design planning phase. Evaluating potential risks as early as possible can help you avoid redesign and additional testing, which can be costly and cause delays.
4. Select appropriate design reviewers. Ensure that at least one independent reviewer is present at each design review, and prepare to defend the reviewer’s competence and impartiality. Actual device users, clinical experts, and supporting staff may also participate in the review process to identify potential problems early in the design.
5. Ensure comprehensive design transfer. Adequately document design transfer. This includes operator training, process setup and validation, work instructions, equipment gaging and calibration, and other activities that ensure design fully translates to production specifications. Read more about how to conduct a medical device design transfer.
Medical device design projects typically involve large amounts of time, resources, and effort to complete. By implementing robust and thorough design control systems, manufacturers can spot design issues early on and avoid potential regulatory risk during an FDA inspection. If you need assistance implementing or managing design controls, please call 512-328-9404 or contact us at info@qaconsultinginc.com.






